Periodic table: Fascinating FAQs
The periodic table is a fundamental tool in the world of chemistry.
It organises all known elements according to their atomic and chemical properties, enabling their behaviour and relationships to be understood in a visual and systematic manner. From students to laboratory professionals, the periodic table facilitates the interpretation of reactions, the formulation of compounds, and the prediction of properties. In this section, we compile curious and interesting questions that help uncover lesser‑known aspects of its history, classification, and key figures.
How many people contributed to creating what we know as the periodic table?
In the 19th century, 63 elements were known, but the lack of consensus on their arrangement led to the First International Congress of Chemists in Karlsruhe, Germany, in 1860. There, Stanislao Cannizzaro presented the concept of “atomic weight,” which influenced Mendeleev and others in organising the elements, laying the foundations for the modern periodic table.
In addition to Mendeleev, other scientists such as Jabir ibn Hayyan, Lavoisier and Marguerite Perey contributed to the development of the periodic table. Today, the table comprises 118 elements.
How many elements does the periodic table have? How are they classified? Let’s bring some order!
A fascinating aspect of the Periodic Table is its capacity to evolve over time. Each newly discovered element requires adjustments to the table, making it a dynamic tool that reflects the ongoing advancement of scientific knowledge. This quality makes the periodic table not only a catalogue of matter, but also a representation of scientific progress.
Did you know?
The way elements are arranged allows the prediction of their properties and reactivity, even for elements not yet discovered.
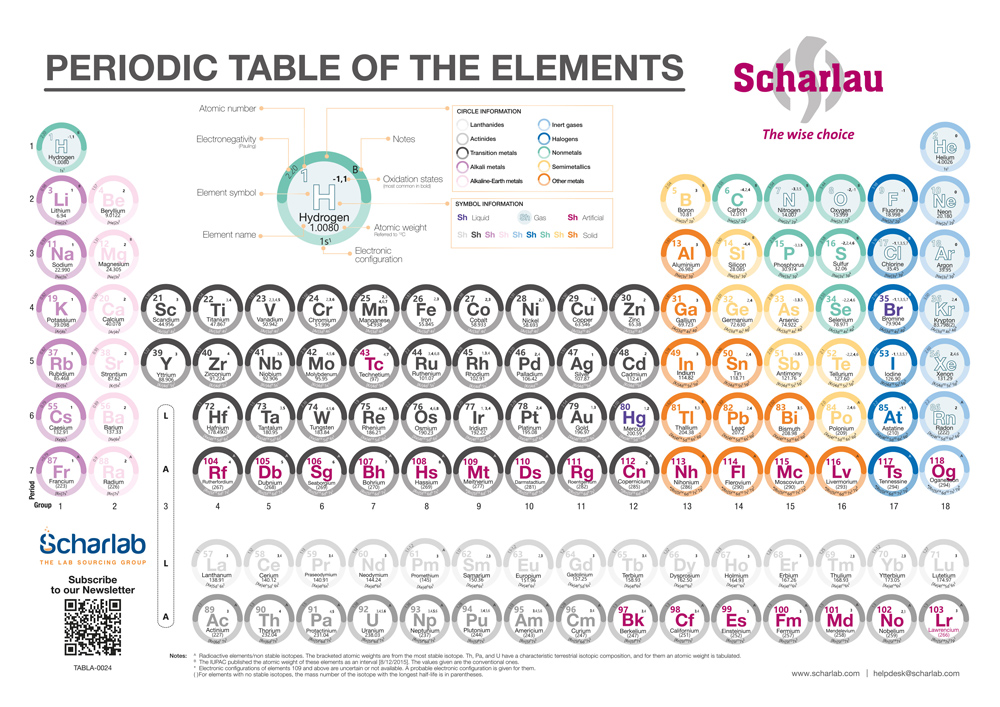
The 118 elements of the Periodic Table are organised in columns (groups) and rows (periods), and are classified into Metals, Metalloids and Non‑Metals. Their distribution is based on atomic number and electronic configuration, which aids their comprehension and ordering. There are 18 groups, and elements within each group share similar physical and chemical properties due to electronic configuration. The period indicates the number of electron shells.
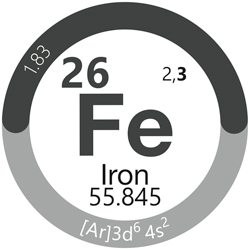
In the periodic table designed by Scharlab, each element group is colour‑coded. For example, metals are coloured dark grey.
Metals
Metals are the most abundant and are subdivided into six subgroups:
- Alkali metals (column 1)
- Alkaline earth metals (column 2)
- Transition metals/ d‑block (columns 3–12)
- Lanthanoids (row 6)
- Actinoids (row 7)
- Other metals (columns 13–16)
Metalloids
There are seven metalloids, located across columns 13 to 16.
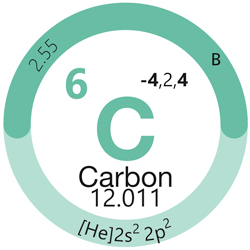
Non-Metals
Non‑Metals are subdivided into:
- Other non‑metals (columns 14–16)
- Halogens (column 17)
- Noble gases (column 18)
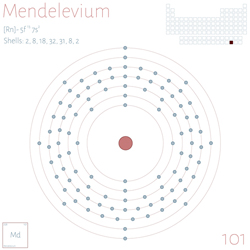
Did you know there is an Element called mendelevium? Do you know what makes it unique?
Yes, you guessed correctly: mendelevium is named after Mendeleev, the creator of the periodic table.
Mendelevium is a synthetic element with symbol Md and atomic number 101. It belongs to the actinoid series and is a transuranic element (heavier than uranium), which means it does not occur naturally on Earth and must be produced in a laboratory.
Mendelevium was discovered in 1955 by a team led by Albert Ghiorso, Glenn T. Seaborg, Bernard G. Harvey and Gregory R. Choppin at the University of California, Berkeley. It was produced by bombarding an einsteinium‑253 target with alpha particles (helium nuclei) in a particle accelerator. Its name honours Dmitri Mendeleev, the creator of the periodic table.
Although its precise appearance is unknown, it is believed to be metallic and silvery in appearance, like other actinoids. Mendelevium has no stable isotopes. Its most commonly studied isotope is mendelevium‑256, which has a half‑life of approximately 1.17 hours, decaying by alpha emission. Due to its short half‑life, all mendelevium isotopes rapidly decay, making them difficult to study or apply. Its production is so challenging that only a few atoms are synthesised in each experiment!
How many elements have been discovered by women?

Francium (Fr) was discovered by Marguerite Perey in 1939 while studying the decay of actinium at the Curie Institute in Paris. It is an alkali metal, with atomic number 87.

Radium (Ra) and Polonium (Po) were discovered by Marie Curie and her husband Pierre Curie in 1898. Marie Curie pioneered radioactivity research and received two Nobel Prizes for her work. These radioactive elements were fundamental to the development of nuclear science.
In addition to Perey and Curie, many other women have made significant contributions to the development of the periodic table and the understanding of elements, although they have not always received equal recognition:

Ida Noddack, a German chemist, co‑discovered rhenium (Re) in 1925 with her husband Walter Noddack. She also proposed the concept of nuclear fission, although her theory was not accepted at the time.

Lise Meitner, an Austrian physicist, collaborated with Otto Hahn on nuclear fission research. Her work was crucial for understanding nuclear decay, leading to the discovery of technetium (Tc). Although Hahn received the Nobel Prize in Chemistry in 1944, Meitner was unjustly excluded.

Harriet Brooks was one of the first women to study radioactivity and collaborated with Ernest Rutherford. She significantly advanced the study of radioactive elements such as radium and thorium, although she was never officially recognised as the discoverer of an element.

Darleane Hoffman and her team synthesised seaborgium (Sg) in the 1990s, confirming the existence of transuranic elements and contributing to their recognition in the periodic table.

 Philippines
Philippines